How Sanofi accelerates treatment for patients in need with CyberGrants for Life Sciences

Overview

Sanofi is an innovative global healthcare company dedicated to chasing the miracles of science to improve people’s lives.
They make this commitment a reality by working to discover, develop, and deliver medicines and vaccines for patients in need. Unfortunately, many patients face barriers to accessing these treatments due to geographic location or financial circumstances.
Customer profile
Product: Bonterra CyberGrants for Life Sciences
Sector: Corporate
Vertical: Life Sciences
To tackle these challenges, Sanofi has implemented a series of humanitarian aid programs to deliver treatments to those who wouldn’t otherwise have access. The Rare Humanitarian Program is the first, the largest, and the longest running program of its kind to treat patients with lysosomal storage disorders — a group of rare genetic conditions caused by enzyme deficiencies.
To learn more about the history of Sanofi’s Rare Humanitarian Program, how it builds sustainable support for patients in need, and how the team at Sanofi uses Bonterra CyberGrants as their medical affairs platform to accelerate product delivery, we sat down with Bonnie Anderson, head of humanitarian programs, and Bill Schwarz, digital product owner and program manager at Sanofi.
How did the program start?
The vision for the Rare Humanitarian Program was first conceived by Henri Termeer, former CEO of Genzyme, in 1991. His belief was that transformative medicines should be accessible to all patients, regardless of their circumstances. This vision became a reality when Genzyme launched the first commercial treatment for Gaucher disease, ensuring its global availability through the program. After Sanofi acquired Genzyme in 2011, it continued to expand the program, now supporting six disease areas with eight products under its leadership.
How do eligible patients access treatment?
Eligible patients can access treatment by contacting a local physician to apply for program inclusion. If eligible, Sanofi approves the product for up to six-month intervals, requiring ongoing monitoring and updates from the physician. This process fosters a strong relationship between patients and their treating physicians.

Today, the program donates 100,000 vials and supports over 1,100 patients annually across more than 70 countries, including patients in war zones and sanctioned countries.
Challenges
How was the program managed before CyberGrants?
Initially, the program relied on manual processes, with applications submitted via mail or fax and reviewed on a monthly basis. Despite moving to SharePoint in 2013 for improved data handling, manual workflows persisted, leading to delays and inefficiencies. The switch to CyberGrants in 2023 revolutionized the application process. Now, physicians can directly submit applications via the Patient Online Referral Tool (PORT), which is powered by CyberGrants, significantly reducing approval times and enhancing data visibility.
What were the biggest pain points of the legacy processes?
Before adopting CyberGrants, the program faced several challenges:
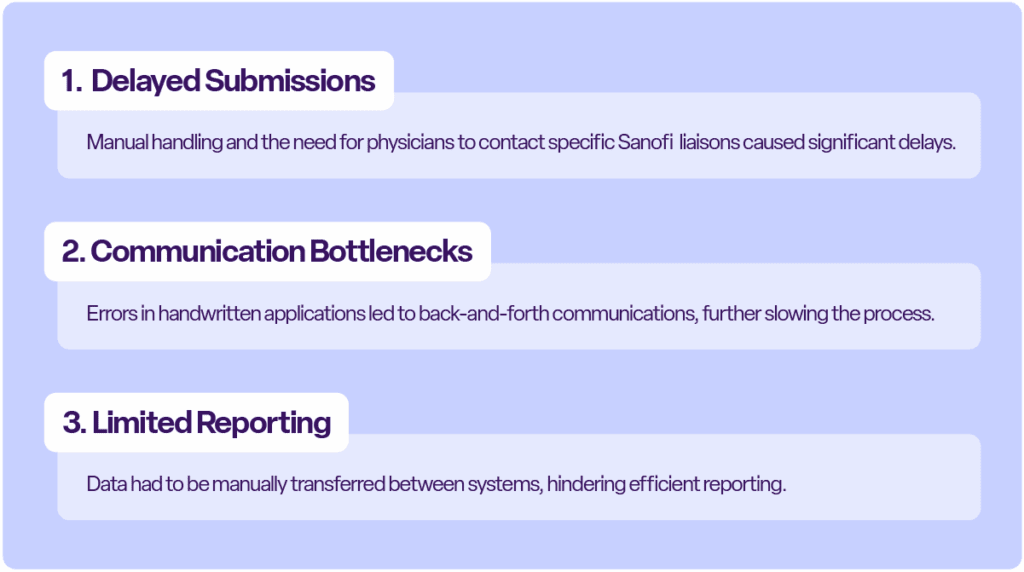
Solutions
How has CyberGrants helped address these pain points?
CyberGrants enabled Sanofi to streamline the application process, allowing physicians to submit applications directly, instead of connecting with a Sanofi medical liaison. Now, if a physician hears about the program, they can log into PORT and directly submit an application on behalf of their patient. The system also introduced automatic reminders to prevent bottlenecks and enhanced visibility for all stakeholders.

The transition allowed Sanofi to revisit application questions, workflow steps, approval criteria, and more to streamline workflows and simplify steps. They also increased compliance by adding a mandatory training component, which requires physicians to confirm a level of understanding of the treatment before approval, strengthening the program and ensuring compliance.

Outcomes
After launching CyberGrants, the first applicant was a physician in Uganda — a new country for the program. From submission to approval, the entire process took just under 36 hours. For the Sanofi team, it was especially exciting to have a new physician in a new country successfully submit an application through PORT.
Sanofi’s commitment to breaking down geographical barriers and providing sustainable treatment options is evident through the continued expansion of the Rare Humanitarian Program. By leveraging CyberGrants, Sanofi is poised to make a lasting impact on patient outcomes globally.
Interested in learning more? Download the full case study.
If you’re a medical affairs grantmaker seeking to optimize and streamline your grant lifecycle, explore how Bonterra CyberGrants for Life Sciences can transform your processes and connect with our experts today.

Work with Bonterra



